RUSTFETISH
Where We forge stories
in rust, silence,
and decay
relics breathe
Rust guides
us through
strange beautyand
silence sharpens
We forge stories
in rust, silence,
and decay
Rust guides us
through
strange beauty
RUST
FETISH
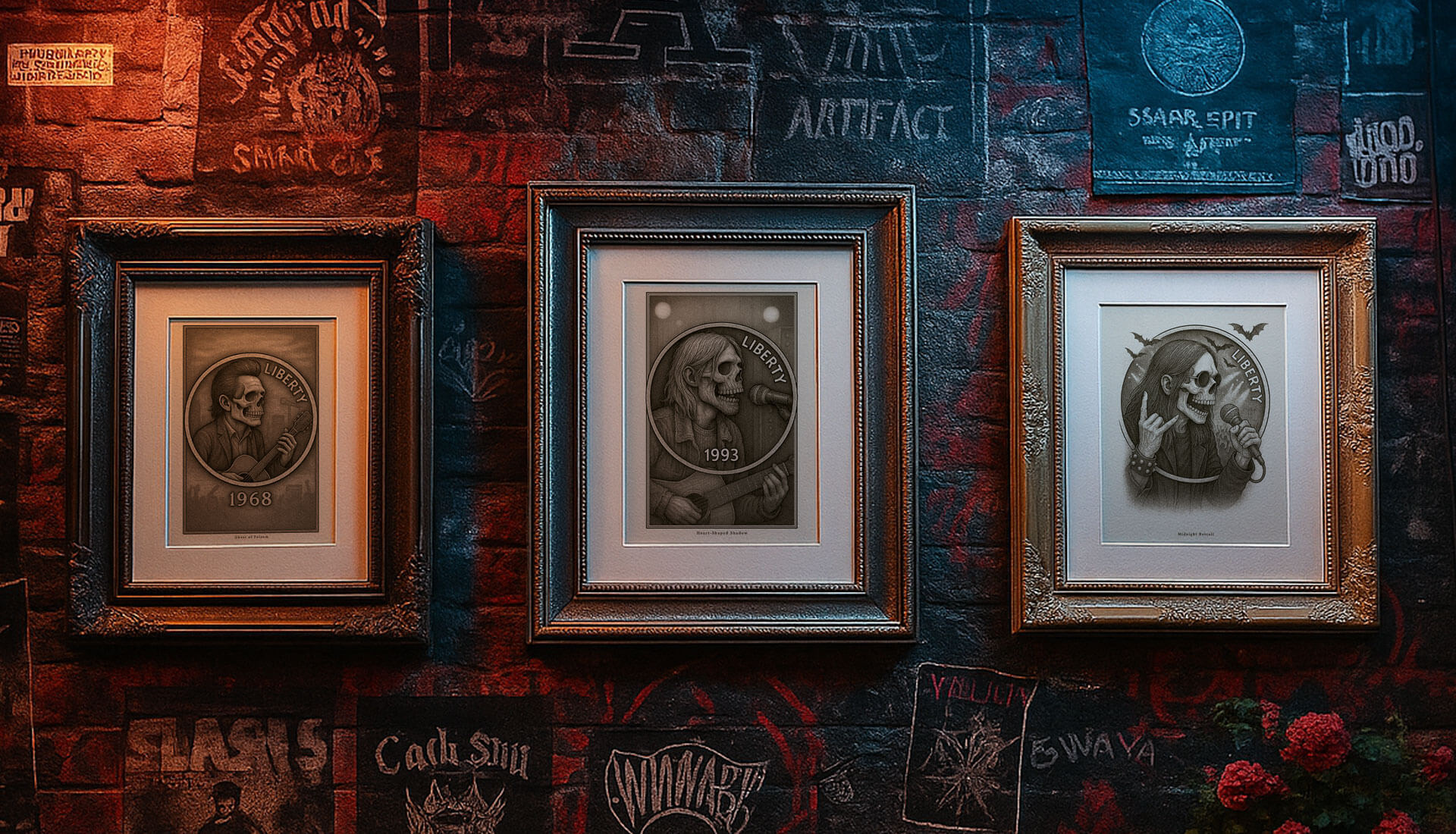
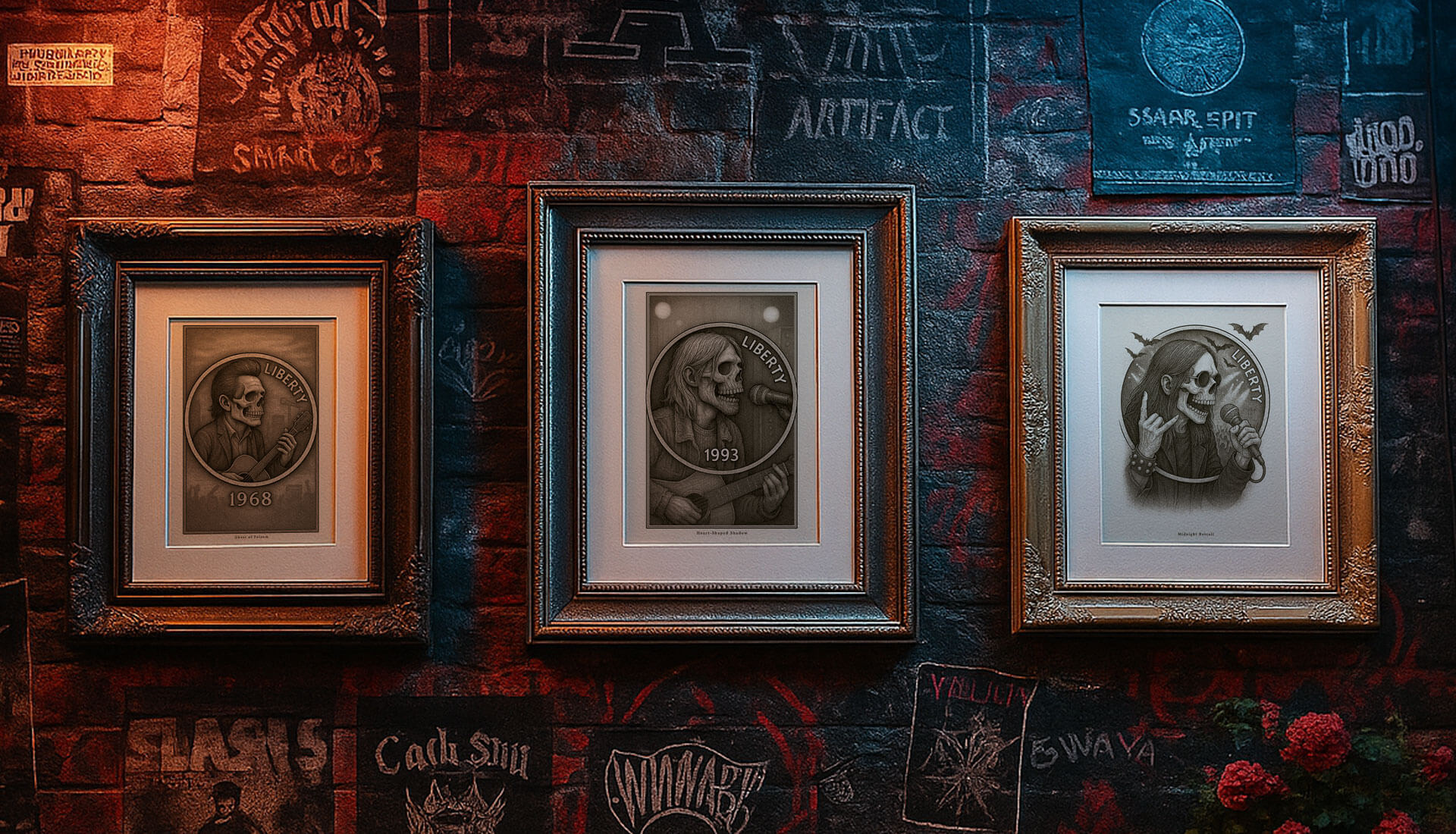
Who we are
This is Rustfetish, where decay is worshipped and the forgotten are carved back into time.

Rust
Not the end, but the beginning. Our work grows from rusted roots and forgotten filigree — where every surface scratches back.
( Corrosion shaped our vision )

Featured Collections
( Books, Prints )
Curtainfall
Engraved echoes from the forgotten circus. This ongoing art series reimagines the Buffalo Nickel as a haunted stage — a coin pressed not with presidents or liberty, but with performers who never left the ring. Books and fine art prints are available for collectors of shadows and sawdust.
( Prints )
Dead Princesses
Royal bones in silver, carved where crowns cracked but never fell. Dead Princesses reimagines the Buffalo Nickel as a fallen throne — sovereigns etched in silence, their legacy unbroken. These prints hold no fairy tales, only rule, memory, and shadow.
( Prints )
Legends of Music
Legends carved mid-riff, not in encore. Legends of Music reimagines Buffalo Nickels as haunted stages — guitars cracked, eyes lit in ghostlight. These prints don’t fade. They echo. Where bone meets silver, the song still burns.
( Prints )
Rustfetish Photography
Where rust breathes, memory clings. Rust Relics captures decay in color — reds, ochres, and iron ghosts worn by time. These prints are oxidized stories. Texture you can almost hear.
THROUGH FLAKE AND FRACTURE
WE’VE HELPED VISIONS CORRODE
INTO SOMETHING UNFORGETTABLE


Ours is a language of decay — scratched in metal, whispered through wire. Not everyone hears it. But you might.
Simply put, we
honor what others
forget
AWARD & RECOGNITIONS
- HOUSE OF DECAY DESIGN PICK (2025)
- OXIDATION HONORS (2025)
- CORROSION CLUB AESTHETICS PICK (2025)
- RUST INDEX DESIGN HONORS (2025)
- BARBWIRE EDITOR'S PICK (2025)
- DARK PATINA FEATURED ART (2025)









